Raman Laser Development
Introduction
Continuous-wave laser sources in the mid-infrared from about 5-10 μm are few and far between. Raman shifting can be used to shift the wavelength of a cw near-infrared laser to reach these desirable regions of the mid-infrared. In a Raman process, an incoming photon scatters inelastically off of a molecule in our Raman gain medium. This causes the molecule to excite to a higher vibrational state while the photon leaves red-shifted. The original photon is a pump photon, while the red-shifted photon is called a Stokes photon. If we pump the gain medium with the pump photons, while at the same time trapping a sufficient number of Stokes photons in the medium, then the Stokes photons can stimulate the Raman process and lasing can occur. The difference in energy between the pump photons and the Stokes photons is the difference between the vibrational energy states and is a property of the material.
Certain crystals have useful properties that can be exploited to achieve Raman lasing more effeciently. Among the relevant crystals, the ones we are most interested in are solid para-H2, and Ba(NO3)2. The main properties that make these crystals so interesting are the large vibrational frequency of the H2 molecules and the large Raman gain coefficients of solid para-hydrogen and barium nitrate crystals. These characteristics especially make solid hydrogen an appealing gain medium for a mid-infrared continuous-wave Raman laser, which would allow our lab to access an elusive yet desirable region of the electromagnetic spectrum for molecular spectroscopy. Therefore, much study has been undertaken on solid hydrogen, including development of a method to grow and crystallize it, measurement of its index of refraction, and initial planning for a proof of concept experiment of a visible solid hydrogen Raman laser. In addition, studies are underway for developing a proof of principle visible Raman laser using barium nitrate as the Raman medium.
Initial Studies with Solid Para-Hydrogen
Molecular hydrogen exists in two distinguishable nuclear spin states: ortho-H2 and para-H2. For Raman lasing applications, pure para-H2 must be crystallized to create the gain medium. To this end, our research group has designed and built a para-H2 converter that uses a ferric oxide catalyst at cryogenic temperatures to convert normal H2 (3:1 ortho:para) produced from a hydrogen generator to 99.99% pure para-H2. The newly purified para-H2 can then be used directly in an experiment, or stored in a Teflon-lined gas cylinder for future use with minimal back-conversion.
In order to design a suitable cavity for the Raman laser, the index of refraction of the solid para-H2 gain medium must be known at low temperatures and at different wavelengths. Therefore, measurements of solid hydrogen crystals were made with the aim of calculating these index values. In these measurements, fresh para-H2 was condensed (see video) and crystallized in a custom cryogenic cell mounted in a liquid He-cooled cryostat under high backing pressure. Optical windows on the cell and the cryostat enabled us to introduce laser beams of different wavelengths through the crystal. The crystal refracted the beams, and by calculating the refractive angle of these beams, we were able to determine the index of refraction for solid para-H2 at these wavelengths. We also studied the index of refraction as a function of temperature and laser polarization. These measurements aided in the subsequent step of cavity design.
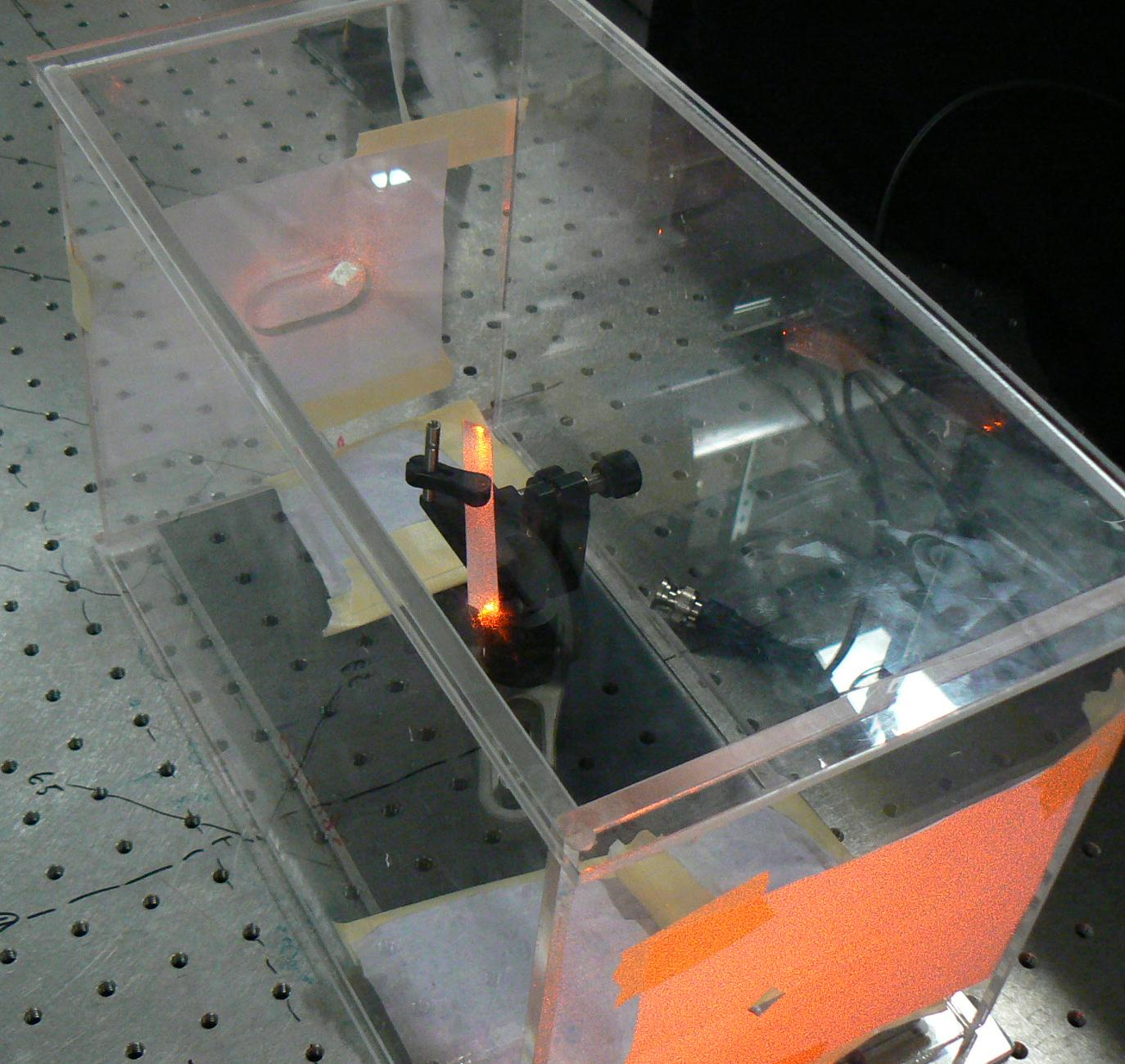
A crystal of barium nitrate illuminated by a dye laser at 610 nm. The crystal serves as the Raman gain medium to convert the dye laser at 610 nm to a laser at 652 nm.
Working with Barium Nitrate
As a proof of concept, we are developing a visible-wavelength Raman laser using barium nitrate. We use barium nitrate because it has the highest Raman gain coefficient of any room-temperature crystal. This allows us to separate the challenges inherent in stimulating Raman lasing from those inherent in dealing with a cryogenic crystal. We are building a doubly-resonant laser cavity, which will collect both the pump and Stokes photons. This will allow us to achieve Raman lasing in barium nitrate with lower pump thresholds than has previously been possible.
Solid Hydrogen Raman Laser
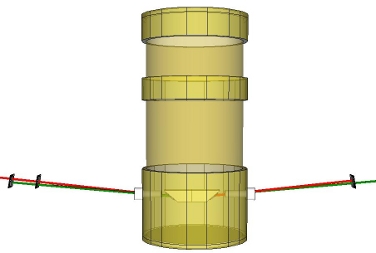
Tentative conceptual diagram of cw solid hydrogen Raman laser. The solid hydrogen cell inside the cryostat converts a pump laser (green) to a Stokes-shifted beam (red). The laser cavity consists of two mirrors that highly reflect at the Stokes wavelength, but transmit at the pump wavelength. A dichroic mirror reflects the pump laser back toward the hydrogen cell, while passing the Stokes beam.
We use the measurements of the index of refraction of solid para-hydrogen to design a cavity for a solid hydrogen Raman laser. Knowing the index of refraction precisely is important in designing the laser cavity so that all interfaces can be made to be at Brewster's angle. This minimizes losses inside the cavity.
Solid hydrogen promises to be a great Raman gain medium because it has a higher Raman gain coefficient than about any other crystal. We will be using hydrogen's huge gain coefficient together with its large Raman shift to reach wavelengths that would be difficult to reach otherwise. Solid hydrogen has been used by many groups to Raman shift pulsed lasers, but we will be using it inside a laser cavity to create a continuous-wave Raman laser. First we will be shifting from green to red in the visible as part of a proof-of-principle. Afterwards, we will use the setup to shift from the near-infrared to the far-infrared.
Related Content
Papers
Talks
Posters
Other Publications
| 22 | L. E. Moore "Production, Crystallization, and Raman Shifting with para-Hydrogen" B. S. Thesis, University of Illinois, 2010. |

